Protective Coating of Crude Oil Storage Tanks
epochem offers a wide variety of epoxy composites and coatings to resurface damaged concrete and stone constructions, protecting them against chemical and environmental attack.
The oil and gas particular field and more particularly the storage tank coating systems are considered in this study. At the beginning, tank corrosion problems are outlined and then a comprehensive theoretical characterization and description for the major types of coatings is presented.
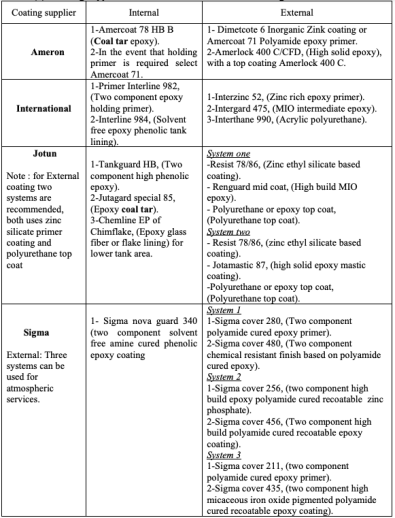
The selection bases are also stated for each type of coating. Then a crude oil floating roof storage tank case study has been undertaken which illustrates the coating selection and discuses the current tank coating problems. In this case study, coating selection was based on two approaches. The first is by referring to the approved coating suppliers, and the second is by referring to current coating specification used by two major oil and gas local companies. Selection
results from the two approaches are then compared and discussed. Research findings are then concluded.
ABSTRACT

1. INTRODUCTION
Steel Storage tanks are used to store fluids such as crude oil, intermediate and refined products, gas, chemicals, waste products, aqueous mixtures, and water. Corrosion is the prime cause of the deterioration of steel storage tanks and accessories; therefore, control and prevention of tank corrosion is of prime importance for efficient plant economics and safety. One of the most efficient methods of tank corrosion prevention is by applying a suitable coating.
Therefore, the aim of this article is to provide up-to-date technological
directions in this field and compare them with what is currently being used by some of the local companies. However, to begin with it was found worth to provide a brief overview of tank corrosion problems which is usually encountered in the oil and gas plants.
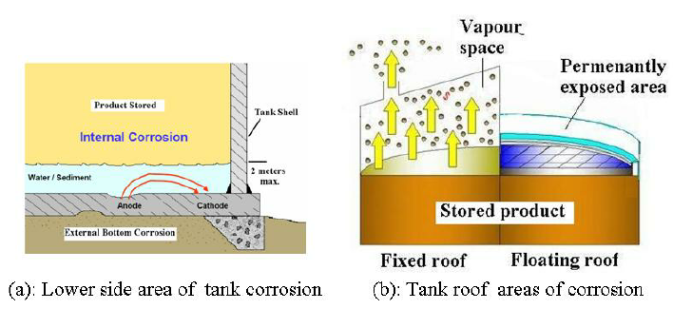
Tank corrosion can either be external or internal. Figure (1)-a, shows the areas of corrosion for lower side of the tank, while Figure (1)-b, shows the areas of corrosion on top side according to the tank roof type. External corrosion of tank bottoms can be significant. The foundation material used for forming a pad under the bottom may contain materials that are corrosive.
For example, cinders may contain sulfur compounds that become very corrosive when moistened. The presence of clay, wood, gravel, or crushed stone as a contaminant in a sand pad may cause pitting corrosion at each point of contact.
Faulty pad preparation or poor drainage may allow water to remain in ntact with the tank bottom. If a tank previously leaked corrosive fluid through the bottom, accumulation of the fluid under the tank can cause external corrosion of the tank bottom. For tanks that are supported above grade, as shown in Figure 1a, an improperly sealed ringwall may allow moisture to accumulate between the tank and the support, thereby acceleratingcorrosion.
External corrosion also occurs when external insulation picks up ground water, or when damaged or improperly sealed openings around nozzles and attachments allow water ingress. Atmospheric corrosion can occur on all external parts of a tank. This type of corrosion may range from negligible to severe, depending on the atmospheric conditions of the locality. A sulfurous or acidic atmosphere can damage protective coatings and increase the rate of corrosion. External surfaces of the tank and auxiliary equipment will corrode more rapidly if they are not protected with paint or other protective coatings or with cathodic protection where surfaces are in contact with moisture. Continuous water contact due to pockets or depressions will be likely to cause localized corrosion. Areas susceptible to this should be coated with coatings designed to withstand immersion. The type of tank and the construction details used can affect the location and extent of external corrosion.
Industrial Chemi Coats
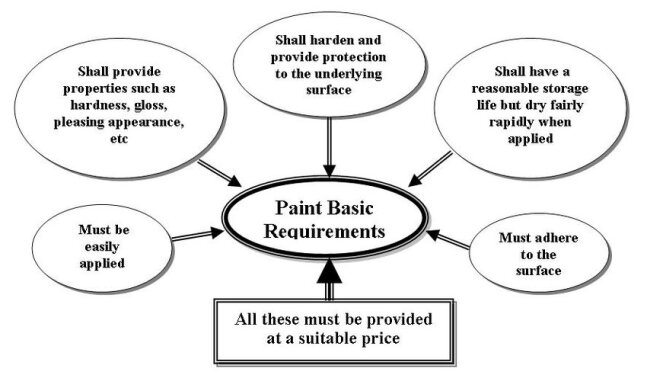
2. PROPERTIES OF PAINTS
Generally, the most obvious properties that a paint must have are shown by the sketch of Figure (2):
Figure (2): Paint basic properties requirements Often, a single paint will not have all the required properties so a number of coats, or
paint system, will be applied rather than a single coat. The first coat or primer must protect and adhere to the tank surface (The paint teeth). The paint film is built up by intermediate coats and a top coat or finishing coat provides protection against sunlight, abrasion, etc and often provides decoration.
Primers and topcoats must, of course, be compatible with one another, and coating suppliers should be consulted for such information. Due to application costs being far higher than material costs, the move is to reduce to a minimum the number of coats in a paint system with a single high-build system. Paints basically consist of solid particles, called pigments, dispersed in a liquid, known as the binder, medium, film-former or vehicle. When the paint dries, the binder binds the pigment particles
together in a coherent film that adheres to the surface.
The binder provides most of the properties required to protect the substrate from the environment, and is the most important constituent of the paint. The binder generally consists of an organic polymer known as a resin dissolved in a solvent. Paints are generally categorized according to the binder type, with names such as alkyd, epoxy, polyurethane, etc. The pigment provides corrosion protection properties along with color to the film (binder). In addition to binder and pigment, most paints contain additional solvent (thinner) to thin the paint out so it can be more easily applied. Solvents completely evaporate and play no part in the dry film. Other additives include extenders, which are similar to pigments and added as a pigment to build up solid constituent or to modify paint properties.
Other additives include driers, anti-skinning agents and thixotropic agents which reduce sagging of the paint when applied to a vertical surface. Paints protect basically by providing a barrier between the substrate and theenvironment. They are electrically insulating which impedes the movement of the ions which take part in the corrosion reactions. Water and oxygen can both diffuse into paint coatings, although some are more impermeable than others and therefore provide greaterprotection.
An inert or barrier pigment such as iron oxide assists in this barrier effect. Leafing pigments, such as micaceous iron oxide (MIO) and leafing aluminum, provide an overlapping, shingle effect, providing a further barrier to the corrosive elements and strengthening the coating. Such pigments also protect the binder from UV egradation. Aluminum is also used for high temperature paints, but should not be used where chemical resistance is required. Such pigments are best used in top coats but can be added to primers. Lamellar pigments will affect the color and appearance of the film.
In addition to the barrier effect, primers usually contain pigments which can provide additional protection. Inhibitive primers contain chemical compounds which dissolve in water to some degree and form a protective passive chemical compound at the anodic sites on the metal. This reduces or prevents corrosion, but such pigments are only used in primers for atmospheric conditions, not for immersed environments where they can give rise to osmotic blistering. Red lead and zinc chromate both provide an inhibitive effect, although primers containing such compounds are no longer used due to their toxicity.
These pigments have now been replaced by zinc phosphate, although this pigment probably provides protection more by barrier than inhibitive effect. Zinc-rich primers provide a barrier effect but, more importantly, have sufficient metallic pigment (90 per cent or more in the dry film) for them to be able to provide cathodic protection. The zinc powder is in sufficient concentration for there to be a conducting path between the zinc particles and the steel. Clearly, the surface must be well prepared so there can be electrical contact between the zinc and the steel. At a scratch in the coating, the zinc corrodes in preference to the steel. Additionally, zinc corrodes at a significantly lower rate than steel and the zinc corrosion products which form tend to block up the pores in the coating providing further barrier protection [3].
YSP-2011 AND 2015 - Epoxy adhesive with mechanical strength and high fatigue resistance
A two component fatigue resistant adhesive, optimized for structural bonding applications requiring high mechanical strength, cleavage and shear resistance. Suitable for typical wed and dry service applications up to 60 C (140 F). for use in original equipment manufacture or repair situations.
YSP 2011 and 2015
CLOSE
YESCHEMI ® FRP are glass fiber reinforced lining systems that can be applied to both steel and concrete substrates and are suitable for chemically and mechanically stressed areas.
FRP laminate systems are used to seal reinforced concrete and recovery basins when storing liquids, indoors and outdoors or as a flooring product. FRP systems are subdivided according to their resin base. These include Vinyl-Ester, Furan, Phenolic and Epoxy resin systems, which are used depending on the particular process conditions.
YESCHEMI
YSP-FRP Lining
CLOSE
سیستمهای پوششی تقویتشده با الیاف شیشه هستند که میتوانند هم روی بسترهای فولادی و هم بتنی اعمال شوند و برای مناطق تحت تنش شیمیایی و مکانیکی مناسب هستند.
سیستم های لمینت FRP برای آب بندی بتن مسلح و حوضچه های بازیابی در هنگام ذخیره سازی مایعات، در داخل و خارج از منزل یا به عنوان یک محصول کف سازی استفاده می شود. سیستم های FRP بر اساس پایه رزینی خود تقسیم بندی می شوند. اینها شامل سیستم های وینیل استر، فوران، فنولیک و رزین اپوکسی است که بسته به شرایط فرآیند خاص استفاده می شود.
